Simplify Medical Device File Management with BPA Software

Automated provisioning of medical device files with Power Automate and BPA software.
Medical device companies need to comply with regulations like ISO 13485, FDA part 11 and MDR to obtain a conformity mark to distribute their products.
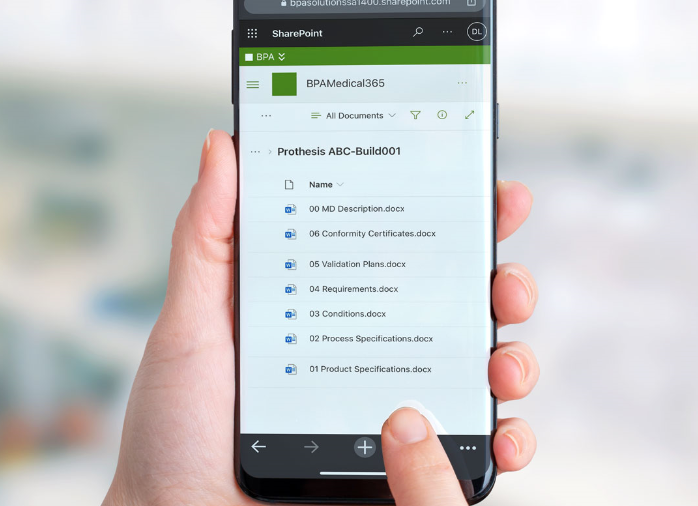
One of the requirement is to establish and maintain medical device files, including medical device intended use and instructions for use, labeling, product specifications, and more.
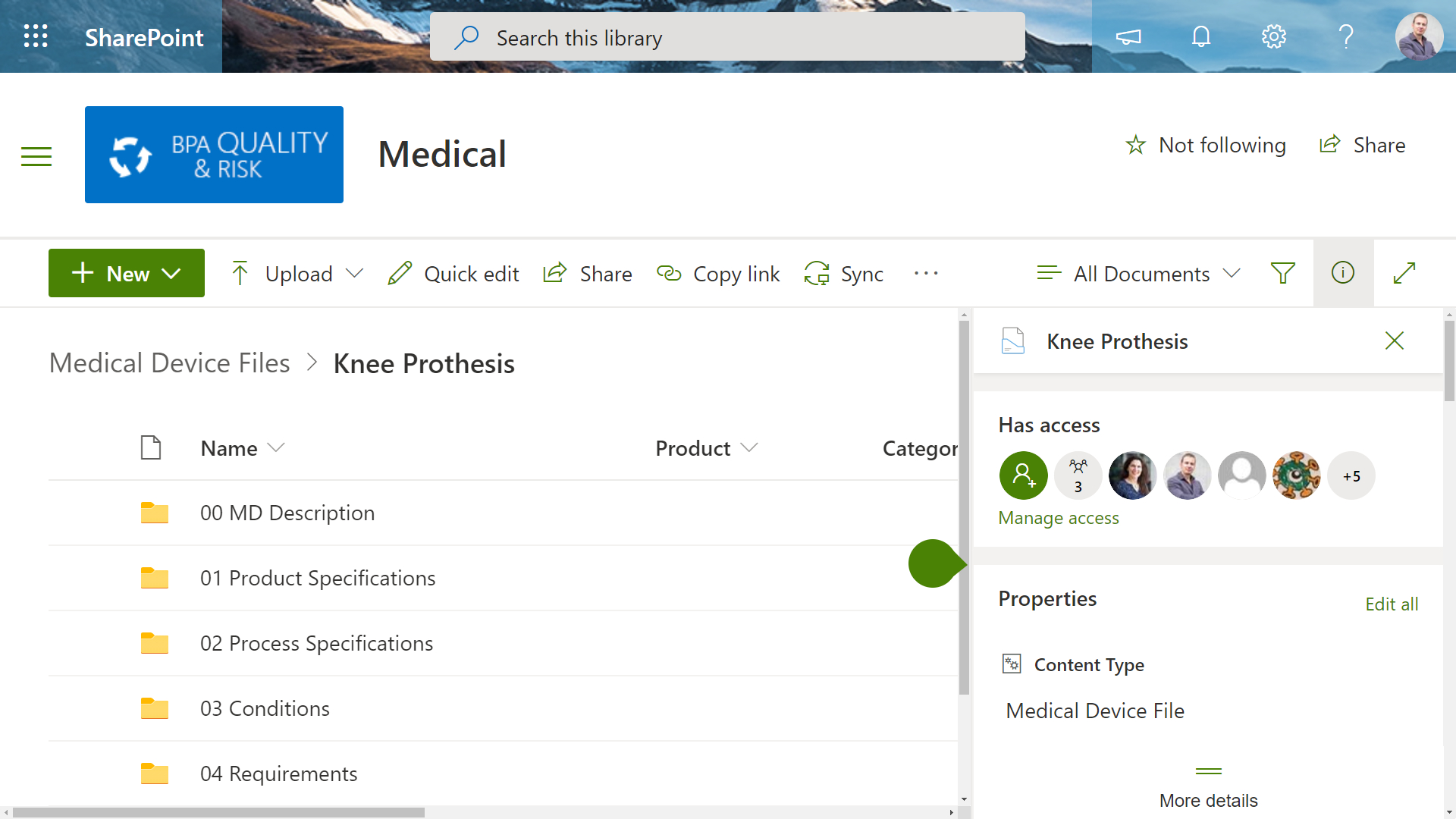
It’s difficult to maintain accurate technical file templates across the whole organization for all products. Each file is usually linked with a specific approval process and the whole document set should inherit some document properties.
BPA has developed a Power Automate workflow to provision medical device files for any product by submitting a simple request. The needed file structure is automatically generated and each file is associated with an independent approval workflow. Automated rules will update file properties with the right product or project.
As a result, organizations have an automated system to generate accurate medical device files with no risk of manual error. Automated rules and approvals drastically reduce time to prepare the needed technical documentation.
The medical device file module can be easily configured with your BPA software on Office 365.




