CFR 21 Part 11 Compliance & Electronic Signature

Title 21 CFR Part 11 is the part of Title 21 of the Code of Federal Regulations that establishes the United States FDA regulations on electronic records and electronic signatures. Part 11 defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records.
In this article we present a simple use case to configure a system for 21 CFR Part 11 compliance.
To handle the “signing signature” referred to in Part 11 documentation, a couple departures from BPA Quality and SharePoint are needed. The first is an e-signature custom field to capture the username and password and the second is a workflow that handles the document approval from start to finish.
Microsoft and SharePoint technologies propose many tools for regulatory compliance, like active directory services, SQL Server database and BI services, SharePoint list and library permission settings, version and audit settings.
In this typical use case, document managers (editors, approvers, distributors) have contributor rights for the document library. Approved documents will be published in another library or site (e.g. company intranet) where end users have reader rights.
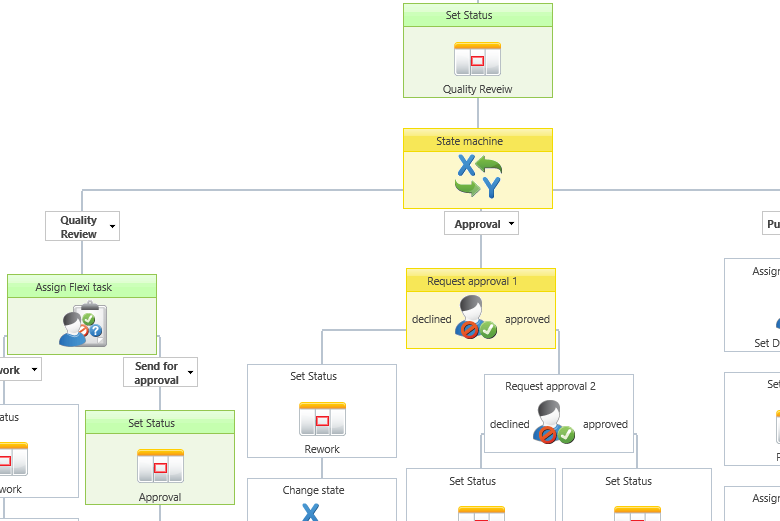
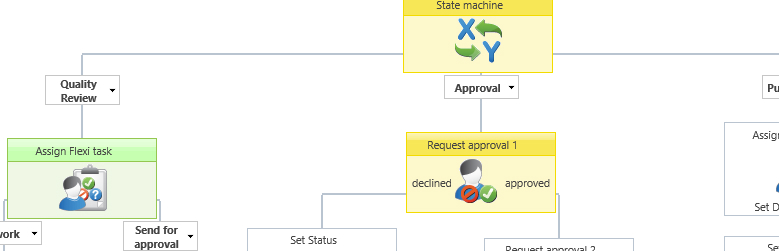
Example of a graphical document approval workflow.
The e-signature module stores, in an encrypted form, the user identity, time and date of signing. This ensures only authorized persons can modify a document, document properties or any quality record. E-signature is required each time documents, document properties or quality records are modified. With no valid signature, a red stamp will be visible in the document or record properties.
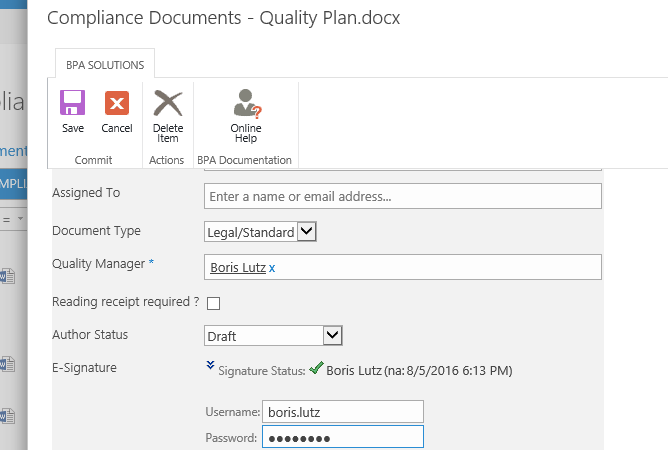
The e-signature custom field in the document properties makes sure the document was approved by the right people prior publishing.
With BPA Quality, end users can easily access published documents from an organizational chart or a process map.
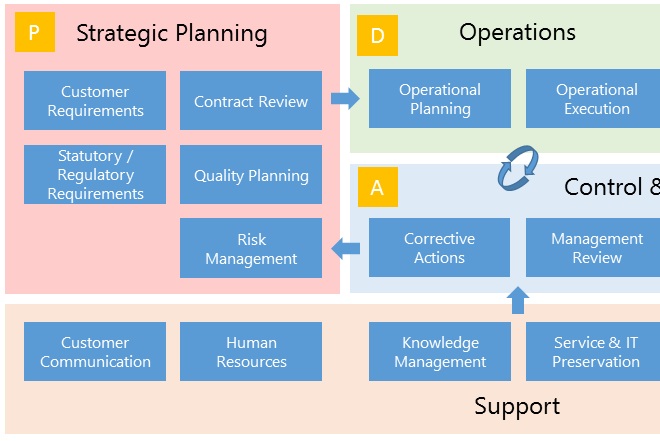
The process map is the ideal entry page for end users to access published documents for the different processes.



