How BPAMedical365 Complies with ISO 13485 ?

Whether you are a medical device manufacturing organization, or a healthcare service provider, or even a supplier of materials or services, the international standard ISO13485:2016 requirements can be applied to establish and maintain a sound quality management system in your organization. The latest edition of the ISO 13485 standard has been released in 2016 and can be considered as the worldwide standard for implementing medical devices quality management systems.
The BPAMedical365 software application can be used by an organization for all stages of the life-cycle of a medical device. BPA has incorporated proactive and reactive tools to identify, evaluate, treat and monitor quality management processes and risks related to the management of medical devices.
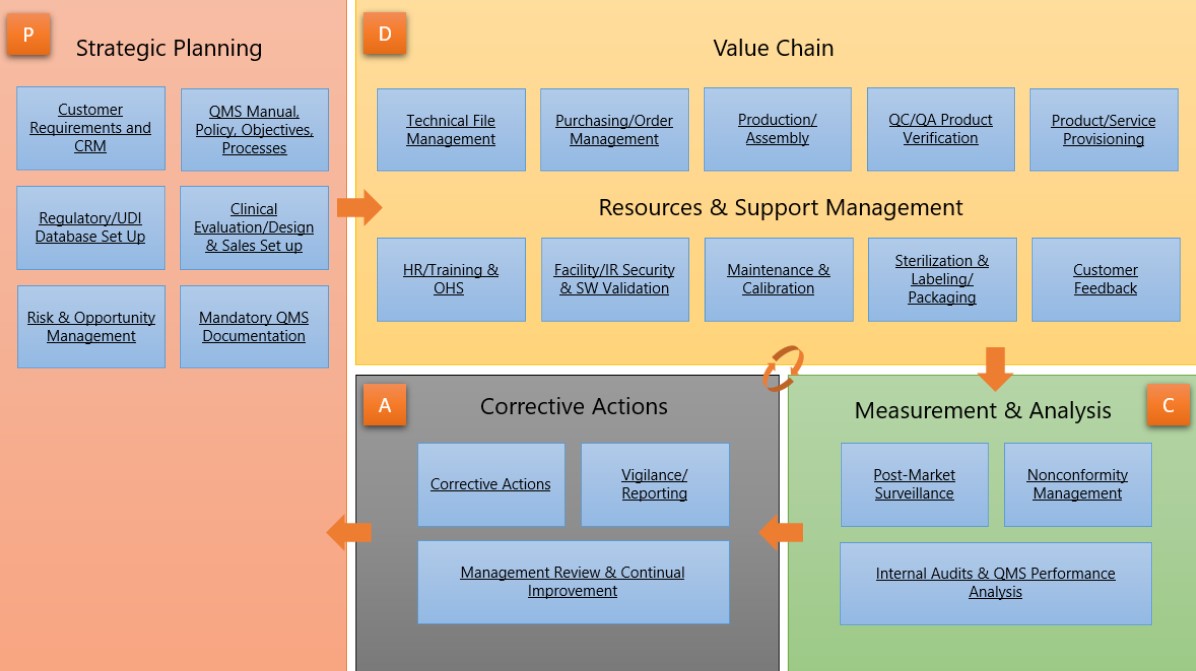
The BPAMedical365 app has a user-friendly navigation grouping all important topics to meet ISO13485:2016 requirements:
|
|
Stakeholder Management
These subjects are part of the ISO 13485:2016 clause to understand your organization and its context. Customer requirements are determined and customer feedback is periodically measured with surveys. Customer requests are tracked in the system. Suppliers are qualified and evaluated periodically with audits and supplier performance indicators. The organization chart provides an easy way for collaborators to understand the organization and access relevant documents. |
|
|
Process Management
As a general requirement for the QMS, processes need to be determined, measured and monitored. The process map makes it easy for collaborators to understand the value chain. List statutory requirements and all applicable regulations (BPA Medical 365 is compliant to many worldwide regulations, such as e.g. MDR and FDA regulations). |
|
|
Knowledge Management
As main documentation requirements, it’s needed to describe, control and manage procedures, documents and records to operate processes. Prebuilt approval workflows including eSignatures ensure documents are approved by authorized persons prior being published. Targeted recipients will need to sign a training receipt when documents are published. Document version history is tracked in the system. Retention policies can be defined for records. |
|
|
Improvement Management
As improvement requirement, it’s needed to continually improve efficiency of the QMS system through quality objectives, audit results, post-market surveillance, analysis of data, corrective actions, preventive actions and management review. The audit module contains prebuilt workflows and power App to simplify audit management, including related actions and audit report generation. As a management responsibility, it’s required to document procedures for management review. Quality objectives are established and their related indicators are periodically measured. Prebuilt workflows and reminder rules ensure corrective and preventive actions are completed timely and efficiently. |
|
|
Risk Management
As a general requirement, a risk-based approach is needed to control processes. Hazards and risks are identified and periodically evaluated based on ISO14971:2019 recommendations. Controls are set to mitigate risks. |
|
|
Design Management
As a documentation requirement, medical device files need to be established and maintained for medical products. Technical files are provisioned and approved with automated workflows, including eSignatures. Design and development changes and their effects on medical files are tracked. Product and process related risks are managed based on FMEA methodology. |
|
|
Competency Management
In the resource management, collaborators shall be competent on the basis of appropriate education, training, skills and experience. List required job competencies and periodically evaluate collaborator competencies to perform their job. Manage the needed training to operate processes. Competency matrix BI reports will be automatically generated for collaborators, jobs and departments. |
|
|
Resource Management
As a resource management requirement, process equipment need to be identified and maintained. Automated workflows and reminder rules make sure equipment are verified and compliant. The information asset register allows to identify and protect sensitive information and data needed to perform operations. |
Built on Office 365, BPAMedical365 not only store data and documents in your secured cloud environment, but also include automated workflows, BI reports, and reminder rules to ensure your QMS remains efficient.
With Teams and SharePoint, our app include communication and discussion capabilities to move towards instant quality improvement for frontline and back-office collaborators on any device.
With AI and machine learning, the app will guide users to find the right documents and find similar events, boosting productivity.