
How Generative AI & ChatGPT simplifies Quality Management

ChatGPT has introduced the use of generative AI with a global success. Generative AI operates machine learning to generate content, such as text and images, based on existing data sources. For instance, a user can interact with a generative AI model by asking questions like, “What can generative AI bring to quality managers?” The model can then generate a summary text based on its trained data.
BPA Pilot leverages generative AI to simplify quality management.
The strategic application of generative AI holds immense promise in the field of quality management, particularly when integrated into an electronic Quality Management System (eQMS). It can rapidly provide quality professionals with targeted information derived from the analysis of both internal and external data sources.
Generative AI can generate synthetic data that closely resembles real data. This can be particularly useful when the availability of real data is limited, or when additional data is needed for training AI models or conducting simulations. For example, the generated data could be easily imported in the eQMS software for testing purposes or end user training.
By learning from a large dataset, generative AI can identify patterns and normal behavior, making it capable of detecting anomalies and outliers. This can be helpful in quality management by identifying deviations from standard processes or identifying defective products.
Generative AI can analyze large amounts of data from equipment sensors and identify signs of potential failures or maintenance requirements. This can help quality managers proactively address issues, reduce downtime, and optimize maintenance schedules. It can further assist quality managers by analyzing data, identifying patterns, and making predictions. This can help identify potential risks and take proactive measures to mitigate them, improving overall product quality and safety.
When included into a cloud-based eQMS, generative AI also facilitates effective communication of recommended actions and helps solving problems by proposing root causes. It represents the next evolution in quality management, offering real-time information and insights for taking strategic decisions quickly.
Prompting the model to get the right information is not trivial. End users need to know about how to ask the right questions to get informative answers. For this reason, having predefined questions in the eQMS based on data points and context is crucial.
The applications of generative AI in quality management are numerous, encompassing areas such as investigations, change management, handling nonconformities and complaints, conducting audits, and managing documents.
BPA Pilot leverages generative AI to simplify quality management and help quality teams and end users to investigate nonconformities, plan actions and propose content to comply with regulations.
Here are a few illustrative examples of how this technology can enhance efficiency, accuracy, and reduce risks and costs, ultimately leading to improved quality, safety, and enhanced operational and financial performance.
A quality manager in a medical device company receives an alert about microbial contamination in a clean room and must ensure compliance with ISO 13485 regulations.
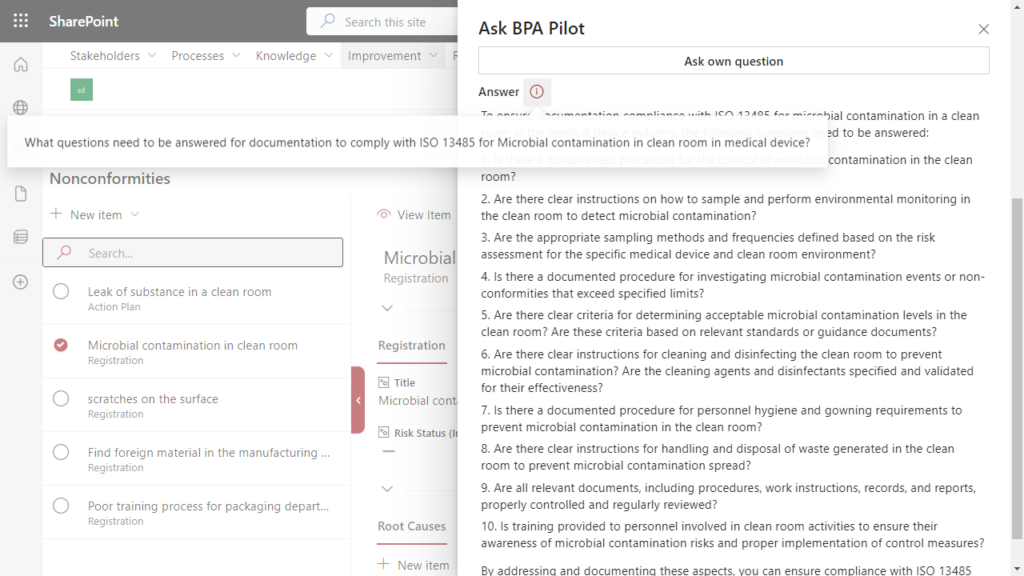
Simplify compliance to ISO 13485 by using generative AI in the context of a nonconformity.
Leveraging generative AI within their eQMS, the quality professional seeks guidance. The technology, having been trained on the latest regulations, assists the user in answering a series of questions required for compliant documentation:
- Does the documentation include a clear and comprehensive description of the clean room facility,
- Is there a documented risk assessment or analysis that identifies the potential sources of microbial contamination in the clean room and the associated risks?
- Does the documentation outline preventive measures and controls to minimize or eliminate microbial contamination
- Is there a written procedure for handling and disposing of any contaminated materials within the clean room?
- Are there documented procedures for conducting routine monitoring of the clean room’s environmental conditions
- Is there a documented procedure for handling and investigating any deviations or non-conformities related to microbial contamination in the clean room?
- Are there documented records of training and competency assessments for personnel involved in clean room activities,
- Is there a documented procedure for periodic maintenance, calibration, and validation of equipment and instruments used in the clean room
- Have internal audits been conducted to assess compliance with the documentation requirements and to identify opportunities for improvement?
Addressing these questions will help to ensure that the documentation for microbial contamination in the clean room complies with ISO 13485 requirements.
Investigating nonconformity root causes is a key step to avoid similar events occurring again. The investigation responsible person has to conduct a root cause analysis because foreign material was found in the manufacturing batch. Generative AI proposes potential root causes to be investigated, facilitating the investigation work:
- Supplier material issues
- Manufacturing equipment
- Human error
- Facility conditions
- Process controls
- Packaging and labeling
- Document control
With this information and by using the 5 why methodology, finding the nonconformity root causes is greatly simplified.
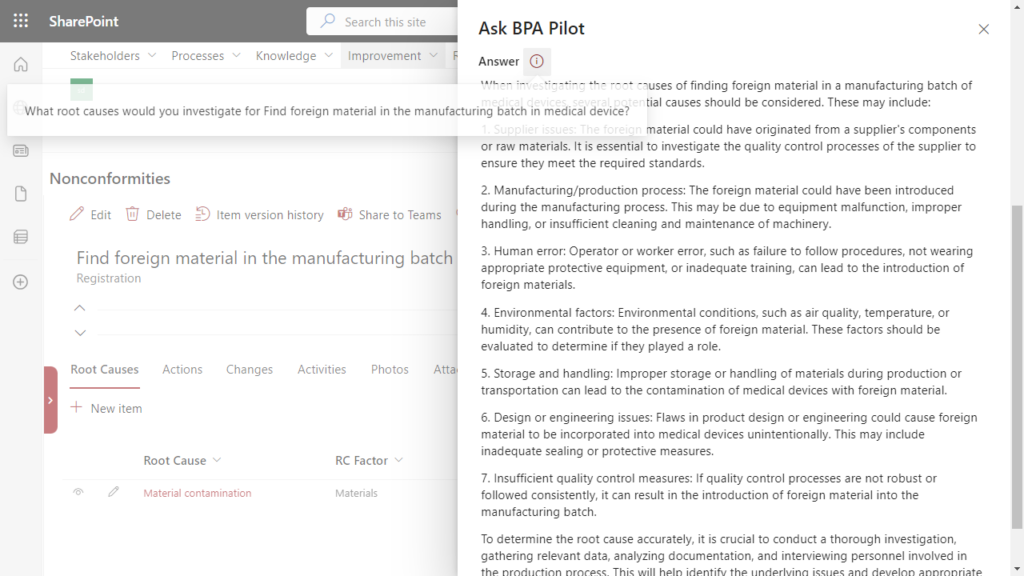
Generative AI helps finding root causes for a nonconformity within the eQMS software.
Policies, procedures and work instructions are the backbone of a quality management system. Writing procedures to comply with regulations is not an easy task. Generative AI helps when writing procedures and propose formatted content to comply with selected regulations. In the case of a procedure for document management to comply with ISO 13485, the suggested procedure would be structured as follow:
- Purpose
- Scope
- Responsibilities
- Document Creation and Approval
- Document Distribution and Access
- Document Control
- Document Review and Revision
- Document Retention and Disposal
- Training and Awareness
- Monitoring and Improvement
Based on the proposed structure and content, the document author has the needed input to write a procedure in compliance with ISO 13485.
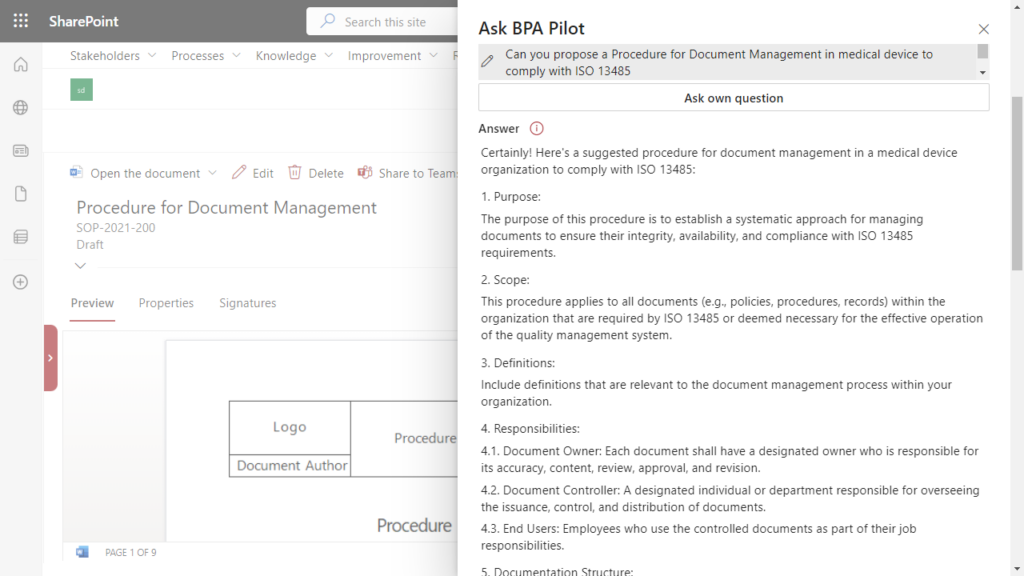
Generative AI supports in writing a procedure by using the BPA eQMS software.
While generative AI holds great promise for quality managers, it’s important to note that these technologies are still evolving, and human expertise and judgment remain crucial for effective quality management.



